China gives conditional approval for Pfizer’s COVID drug Paxlovid

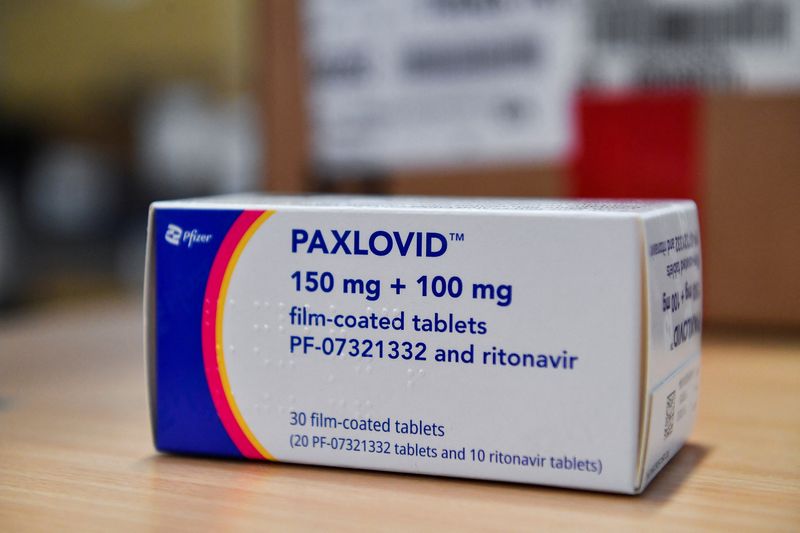
© Reuters. FILE PHOTO: Coronavirus disease (COVID-19) treatment pill Paxlovid is seen in a box, at Misericordia hospital in Grosseto, Italy, February 8, 2022. REUTERS/Jennifer Lorenzini
BEIJING (Reuters) – China’s medical products regulator said on Saturday it has given conditional approval for Pfizer (NYSE:)’s COVID-19 medicine Paxlovid to treat adults with mild to moderate disease who have high risk of progressing to a severe condition.
The National Medical Products Administration said further study on the drug needed to be conducted and submitted to the authority.
Fusion Media or anyone involved with Fusion Media will not accept any liability for loss or damage as a result of reliance on the information including data, quotes, charts and buy/sell signals contained within this website. Please be fully informed regarding the risks and costs associated with trading the financial markets, it is one of the riskiest investment forms possible.
Published at Sat, 12 Feb 2022 01:50:29 -0800