Bluebird Bio: Gene Therapy Launches Could Bring Big Revenues, Providing They Meet Approval
Bluebird bio is a major player in gene therapy for haemoglobinopathies Shutter2U/iStock via Getty Images
Bluebird Bio (BLUE) is a biotech company specialising in lentiviral vector gene therapies for genetic blood disorders. Its portfolio offers potentially curative treatments for conditions such as beta-thalassaemia and sickle cell disease, a previously unheard-of possibility. A previous approval in Europe, however, was brought to a swift end over failure to convince German payors the treatment was worth the $1.8 million price tag.
With upcoming product launches, 2022 could be an exciting year for Bluebird. Their novel therapies are likely to be high profile due to both their potentially curative effects and large price tag, meaning a successful launch would bring with it lots of publicity. However, for a company that doesn’t have any approved treatments, failure to gain approval would be significant drawback.
Catalysts
2Seventy spinoff
Bluebird closed 2021 with the spinoff of its oncology program into a separate entity, 2Seventy Bio. With it, 2Seventy takes $442 million in cash as well as the immune-oncology portfolio that includes Abecma, a fifth-line CAR-T therapy for multiple myeloma, approved by the FDA earlier in 2021. This was touted as a way to refocus priorities, with each company now # “laser focused” on its speciality without having to compete for resources, according to CEO Andrew Obenshain.
This makes practical sense, but there is an element of risk in splitting from the part of the company with an approved drug. Bluebird is an ambitious company, with the potential to be developing revolutionary drugs that could bring in strong revenues in the next few years, but this is dependent on first getting approval of their candidate products.
Ambitious pipeline nearing launch
Bluebird’s immediate pipeline consists of three gene therapies nearing approval in the US., making 2022 and early 2023 a critical period. Successful approval for each product would position Bluebird as a leader in the gene therapy landscape, which currently only includes one other approved treatment. On the other hand, there remains the risk of further delays or FDA rejections.
The first product, Lenti-D (eli-cell), treats cerebral adrenoleukodystrophy (CALD), a very rare neurological disease affecting only around 50 patients in the US for whom there are very few alternative options. The other two treatment are both for haemoglobinopathies: beti-cell for beta-thalassaemia and lovo-cell for sickle-cell disease, each of which are significant causes of patient burden in the US.
While Bluebird has been hoping for a speedy approval of their first two products, they are still facing delays. The FDA initially accepted beti-cell for priority review in the US, with a decision expected by May 2022. However, they have since announced this will be pushed back until August 19, 2022, in response to a regulator request to review further documents from Bluebird. Meanwhile, the PDUFA date for eli-cell of 17 June has been pushed to September 16, 2022. These delays should not impact the priority review status, with both drugs having been granted orphan drug, breakthrough therapy and rare paediatric disease status meaning there remains important clinical need for the treatments.
The delays are not altogether unsurprising, considering the complex and novel nature of the drugs; beti-cell and eli-cell are in line to become the first lentiviral vector gene therapies for rare genetic disease in the US. However, other than the three above products, Bluebirds portfolio is still in the early stages of clinical development, several years away from any additional launches to bring in revenue.
Immediate portfolio estimated launch dates
|
Product |
Approval status |
|
Eli-cell (NASDAQ:CALD) |
16 September 2022 (delayed from June 2022) |
|
Beti-cell (beta-thalassaemia) |
August 19 2022 (delayed from May 2022) |
|
Lovo-cell (NYSE:SCD) |
Submission planned Q1 2023 |
Source: Fierce Pharma 2022; JP Morgan Annual Healthcare Conference
Portfolio in detail
Eli-cell (for cerebral adrenoleukodystrophy)
Eli-cell would be the first of Bluebird’s gene therapies approved in the US. Indicated for the very rare cerebral adrenoleukodystrophy (CALD), eli-cell offers a much-needed treatment option. While it is not likely to be the major revenue source with only a small patient pool, it would be a good first step for Bluebird to build awareness and education around gene therapy.
Beti-cell (for beta-thalassaemia)
Beti-cell was first launched in Europe as Zynteglo, receiving European approval in June 2019. It was touted as a revolutionary drug launch as the first gene therapy option to treat beta-thalassaemia with estimated sales of $1.87 billion by 2024.
However, the launch suffered a series of setbacks with manufacturing difficulties delaying the initial launch, as well as the admittedly unavoidable pandemic difficulties. Furthermore, shortly after treating its first patient in February 2021, Bluebird announced withdrawal from Germany after failing to reach reimbursement agreements over beti-cell’s $1.8 million price tag. Germany had initially offered up to $950,000 for the treatment if it was still working after five years, in line with the expected launch price. Bluebird’s subsequent demands for almost double this was simply too great for German payors to accept.
Bluebird ultimately took the decision to leave the European market altogether, instead moving focus to the US. While the CEO Andrew Obenshain blamed the Europeans for failing to recognise the innovation, the eye-watering price-tag means Bluebird must convince US payors of the treatment’s value, and hope insurers are willing to offer it.
While still rare, beta-thalassaemia represents a larger patient pool compared to CALD, with Bluebird estimating there to be around eligible 1,500 patients in the US. While Bluebird have not released the price of the treatment in the US, these patients could bring in a significant cash windfall.
The need for beti-cell
Beta-thalassaemia is an inherited condition that disrupts haemoglobin production, causing fatigue and anaemia. It is a rare but serious condition, resulting in a five-fold increased mortality rate and 23 times higher healthcare costs than the general population, according to Obenshain in his JP Morgan presentation. Currently treatment is based around regular blood transfusions, coupled with further drugs to control iron levels, meaning it constitutes a serious burden to patients.
Beti-cell aims to be a one-time, potentially curative treatment. In clinical trials is has shown good efficacy with around 80% of patients no longer requiring blood transfusion. For these patients, beti-cell could mean a dramatic improvement in quality of life. Importantly for payors, this could also mean dramatic reductions in future costs that may justify the high initial costs.
Driving value
As seen with the pricing difficulties in Europe, the significant price-tag of beti-cell could well be a stumbling block. While evidence shows its efficacy, the high cost may simply be too much for healthcare systems to accept. Mr Obenshain stated in the presentation that they are confident payors see the value in this one-time treatment, but it will remain to be seen if they can put this into practice. However, Bluebird already has sales teams and education programs in place ready to start promoting beti-cell and gene therapy, ready for launch.
With the launch date fast approaching, Bluebird are approaching the moment of truth of whether their products can start bringing in revenue. However, there can be a 90 – 120 day period from patient enrolment to treatment, meaning it will take time to build up real-world evidence to support claims of efficacy and build physician confidence.
Lovo-cell (for sickle cell disease)
Lovo-cell is the third of Bluebirds major releases, a gene therapy for treatment of sickle cell disease. With over 100,000 patients in the US, this would likely be the biggest of Bluebirds trio.
Sickle-cell disease is a genetic condition resulting in malformed red blood cells that can lead to blood vessel blockages reduced red blood cell function. Current treatments focus on relieving symptoms, but there is significant mortality and morbidity associated with SCD, with 25% of patients experiencing a stroke by age 45, and median age of death remaining in the 40s.
Bluebird is hoping to complete their validation stages by end of 2022, with submission planned for Quarter 1 2023.
Like the other two treatments, lovo-cell will not be cheap, although Bluebird hope to leverage the groundwork of the beti-cell launch, such as education around gene therapies and relationships with physicians. There is also the added benefit that around two-thirds of physicians who treat beta-thalassaemia also treat SCD, putting Bluebird in a strong position to further leverage learnings, with contracts already in place between payors and Bluebird to facilitate onboarding.
As with many of Bluebird plans, unfortunately there have been hurdles with submission. In December 2021 the FDA placed the program on partial clinical hold for patients under the age of 18 after one patient developed anaemia. While this should not affect other aspects of the submission, it is another headache they must deal with. During the webcast, the Bluebird team was confident that the anaemia was not due to treatment, although they are still evaluating potential causes. Regardless, BLA submission is still planned for Quarter 1 2023.
Other safety concerns were raised after two patients developed cancer in clinical trials. While efforts are ongoing to pinpoint the exact cause of these cancers, this may not be the end due to the severe prognosis of SCD and the important need for a the potential curative treatment.
Long term plans
In addition to the product launches, Bluebird is pursuing manufacturing optimisation programs to help reduce costs. Research into suspension lentiviral vectors (LVV) are hoped to significantly increase yields and reduce per patient cost by a massive 90%, with planned launches in 2023 for lovo-cell. Bluebird are also investigating cryopreservation methods to reduce costs. Should these efforts be successful, it would allow Bluebird further savings while also allowing for a price reduction to improve accessibility of the treatment.
Risks
The competitive landscape
Bluebird remains a leader in gene therapies and are among the first to bring a finished product to market. Mr Obenshain explained that while there are competitors investigating gene therapies for haemoglobinopathies such as SCD, they are still in early development and a long way off to launch. Furthermore, with over 100,000 SCD patients in the US, there is plenty of room for numerous treatments meaning competitors are unlikely to disrupt immediate plans.
Vertex Pharmaceuticals (VRTX) have announced a $900 million deal with CRISPR Therapeutics for further control of their gene therapy for beta-thalassaemia, making it a direct competitor for beti-cell. While this is no means a hammer blow to Bluebird’s success, with beti-cell on track to reach approval first, Vertex have the advantage of size and experience as a player in the rare disease market. Bluebird must therefore count on convincing payers of the advantage of viral vector gene therapies over CRISPR, and getting patients enrolled onto treatment and creating the valuable real-word evidence needed to convince physicians.
Demonstrating value
There is not denying that gene therapies are extremely expensive, even compared to modern targeted therapies and an industry characterised by ever-increasing drug costs. Bluebird have already shown willingness to quit markets if pricing agreements are not met. However, Mr Obenshain was confident they have learnt from this and that payers will recognise the value of a one-time treatment, which has been accepted for priority review in Europe.
Approval failure
Possibly the key consideration about Bluebird’s financial position is the lack of material revenues from their products. The short time in Europe did not lead to meaningful revenues, and these will not arise in the US until approval. Furthermore, with a significant accumulated deficit of $3.56 billion as of September 2021, Bluebird are in need of revenues. Failure to successfully launch key products could significantly hamper efforts to become profitable, particularly with other pipeline product still in early stages. The novel nature of these therapies also means that unexpected adverse events, manufacturing woes or regulatory changes could easily limit success. Bluebird’s portfolio uses a novel lenti-viral mode of action. Should FDA take serious issue with the delivery method, there could be a chain-reaction of delays across all products. While there is no current suggestion that this is necessarily likely, it is a risk that must be considered.
Financials
Bluebird are currently sitting on $440 million of restricted cash, cash, cash equivalents and marketable securities of $442 million, with an anticipated cash burn of <$400 million for the next year. They are also aiming to raise another $150 – 200 million in non-diluted cash flow through beti-cell and eli-cell review vouchers. Furthermore, Bluebird have enjoyed a surprise cash saving of $120 million per year from real-estate costs through its new offices, thanks to a hybrid working style and many of its staff able to work from home.
In respect to cash, Bluebird should then be able to comfortable weather at least the next year. With an estimated cash supply of >$600 million available from cash reserves, savings and voucher sales. Furthermore, as a company free of debt, it is in a relatively strong secure position to see itself through until its portfolio starts bringing in revenue of its own.
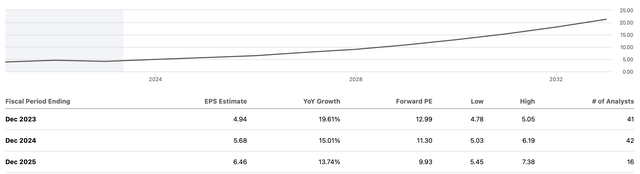
Bluebird has been predicted to see a 37% increase in revenue, for a predicted 24% annual earning growth. While this is dependent on successful product launches, it is a positive and reassuring prediction.
Bluebird is currently trading at $6.85 (03/02/2022), down from around $30 this time last year. The stock has seen a fairly steady decline in price over the past few years, which has not been helped by numerous delays and clinical trial holds over the years, including the temporary halt on sickle cell disease trials in February 2021 that correlated with a price fall from $30 to $16. But with major product launches approaching, 2022 has potential for the company to begin bringing in profits and seeing a share price turnaround.
Conclusions
Bluebird Bio has had some difficult few years, with many hurdles faced along the approval journey. There are still challenges ahead, as made all too clear by the further recent delays in the FDA approval dates. But there is no denying that they could be on the verge of bringing revolutionary and potentially curative treatments to patients with severe genetic diseases that previously had little option than regular blood transfusions.
There is much riding on the success of this year. Failure to receive approval could put Bluebird in a difficult position for the future, whereas success would likely bring with it strong revenues over the next few years and a welcome long-awaiting share price rise.
Published at Sun, 06 Feb 2022 17:33:41 -0800